On This Page:
The limbic system, a complex network of interconnected brain structures, plays a pivotal role in regulating various aspects of human behavior and emotion while also contributing to the processing of memory and motivation.
This neural system encompasses the amygdala, hippocampus, thalamus, hypothalamus, and other structures, all working together to manage emotional responses, especially those tied to survival instincts, social bonding, and memory consolidation.
These functions include but are not limited to emotional processing, memory formation, motivation, and the regulation of essential physiological functions such as stress responses, feeding, reproduction, and the fight or flight response.
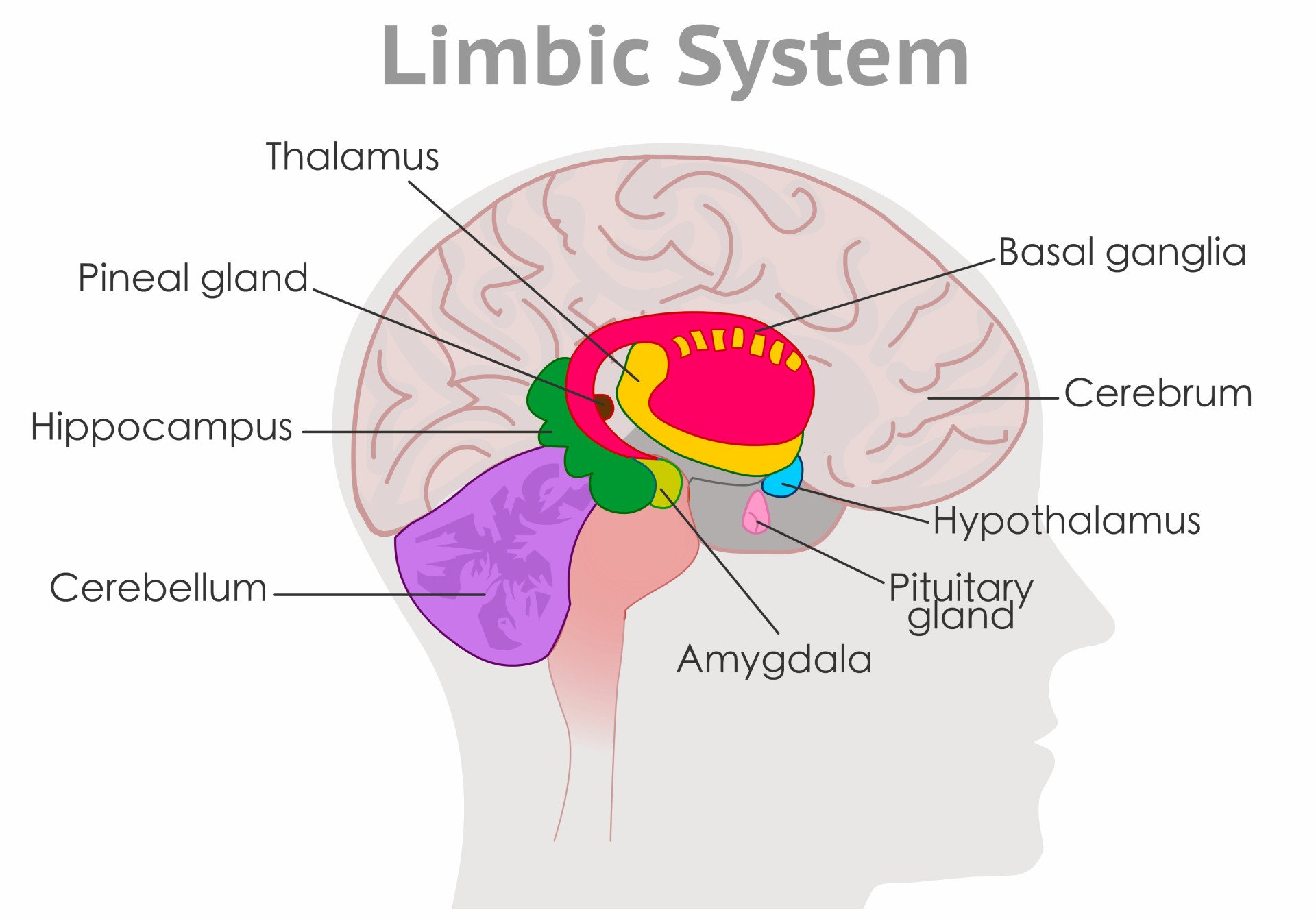
Where is the limbic system located?
The limbic system is located within the cerebrum of the brain, immediately below the temporal lobes, and buried under the cerebral cortex (the cortex is the outermost part of the brain).
Limbic system function
The limbic system was originally called the rhinencephalon (meaning ‘smell brain’) because it was thought to primarily involve the sense of smell.
Psychologists now recognize that the limbic system serves many more functions than previously believed. These structures are known to be involved in processing and regulating emotions, forming and storing memories, sexual arousal, and learning.
The limbic system is thought to be an important element in the body’s response to stress, being highly connected to the endocrine and autonomic nervous systems.
The nerve cells (neurons) within the limbic system are structured differently from those in the cerebral cortex. In the cerebral cortex, the cells are primarily neocortical, meaning they are formed into six layers.
Within the limbic system, the cells are either arranged in fewer layers or are more jumbled. As there is less complexity of the cells within the limbic system, this has led people to believe that this system is evolutionarily older than the cerebral cortex itself.
Hippocampus
There are two hippocampi located in each hemisphere of the brain. They are seahorse-shaped and are structures mainly associated with being the memory centers of our brains.
Episodic memories are formed in the hippocampus and then filed away into long-term storage throughout other parts of the cerebral cortex.
The hippocampus plays a role in spatial navigation and has also been associated with learning and emotions (Tyng, Amin, Saad, & Malik, 2017).
This area also has widespread connections to brain regions involved in cognition and movement control (McEwen et al., 2016).
The hippocampus is also known as a site where neurogenesis occurs – this means that new nerve cells are made here from adult stem cells.
Damage to the hippocampus
Due to the hippocampus’s involvement in memory, damage to this area can lead to severe memory impairments.
Damage can also be detrimental to spatial memory, for instance, remembering directions to locations that should be familiar to the individual.
Damage to the hippocampus can disrupt cognitive functions such as learning, memory, and spatial navigation and contribute to dementia symptoms like memory loss, disorientation, and confusion (Gulyaeva, 2019)
In Parkinson’s disease, hippocampus damage can worsen cognitive symptoms like executive dysfunction, visual-spatial deficits, and memory problems that many patients experience (Xie et al., 2011).
Structural and neurochemical abnormalities have also been found in the hippocampi of young people with bipolar disorder (DelBello et al., 2006).
Amygdala
The amygdala is an almond-shaped structure located right next to the hippocampus. The main function of the amygdala is in emotional responses, including feelings of happiness, fear, anger, and anxiety.
This area is also key for the formation of new memories. The amygdala interacts with the hippocampus by attaching emotional content to memories.
It has a role in how memorable memories can be – memories with strong emotional components tend to stick rather than those with little emotional content.
‘Fear learning’ is also an element of the amygdala.
Fearful memories can be formed after only a few repetitions, which can result in avoidance of certain fearful stimuli. Therefore, the amygdala is linked with the fight-or-flight response, as stimulating activity can influence the body’s automatic fear response.
In recent years, it has been discovered that along with the hippocampus, neurogenesis also occurs in the amygdala, meaning that new neurons are created here and expanding our knowledge of its role in the limbic system (Jhaveri et al., 2018).
Damage to the amygdala
Damage to the amygdala may result in more aggression, irritability, loss of control of emotions, and deficits in recognizing emotions, especially recognizing fear.
Damage to both sides of the amygdala can result in fewer feelings of shame about breaking social rules as well as trouble recognizing fearful and shamed facial expressions correction. This suggests that the amygdala may help detect unclear social situations (Piretti et al., 2020).
Reduced amygdala volume may underlie vulnerability to stress and depression. A study found that childhood violence exposure was linked to reduced amygdala volumes, which interacted with later life stress to predict worsening depression over time (Weissman et al., 2020).
Structural and neurochemical differences in the amygdala have been found in young people with bipolar disorder, suggesting an association between amygdala volume and this disorder (DelBello et al., 2006).
Cingulate Gyrus
The cingulate gyrus is part of the cingulate cortex of the brain and is thought to be an integral part of the limbic system.
This area is believed to help regulate emotions, behavior, and pain, as well as being responsible for controlling autonomic motor function.
This area is thought to involve fear and the prediction and avoidance of negative stimuli through monitoring the body’s response to unpleasant experiences.
Damage to the cingulate gyrus
Damage to the cingulate gyrus can result in emotions being inappropriate, having a lack of fear, impaired sense of pain, and learning impairments.
This region has also shown differences in structure in those with Autism, depression, obsessive-compulsive disorder, posttraumatic stress disorder, and bipolar disorder due to its role in emotional processing (Yucel et al., 2003).
There is thought to be reduced volume and altered activity in the anterior and posterior cingulate cortex in those with schizophrenia (Ponirakis et al., 2022).
Likewise, there have been reduced gray matter volumes in the anterior cingulate cortex of people with ADHD (Carmona et al., 2005).
Hypothalamus
The hypothalamus’ most basic function is homeostasis (maintaining a steady internal state).
This region controls most autonomic functions, such as hunger, thirst, body temperature, blood pressure, heart rate, and sexual activity.
The hypothalamus also serves as an interface between the nervous system and the endocrine system and in the regulation of sexual motivation and behavior.
The hypothalamus also has a role in controlling the body’s response to stress. To control these many functions, the hypothalamus integrates information from other parts of the brain and is responsive to a variety of stimuli, such as light, odor, stress, and arousal.
Damage to the hypothalamus
Damage or abnormalities in the hypothalamus have been linked to several mental health conditions, including anxiety, depression, bipolar disorder, aggression, and obsessive-compulsive disorder. (Herman et al., 2016).
This may be because hyperactivity in the hypothalamus can lead to excessive anxiety and agitation, whereas underactivity can contribute to depression and lack of motivation.
Chronic stress and elevated cortisol levels associated with hypothalamic dysfunction may predispose some individuals to mood disorders. (Herman et al., 2016)
Differences in hypothalamic-pituitary-adrenal (HPA) axis responsiveness related to genetic factors or exposure to early life stress can make some people more vulnerable to PTSD and mood disorders later in life. (Pagliaccio et al., 2015)
Basal Ganglia
The basal ganglia are a group of structures situated at the base of the forebrain and the top of the midbrain.
Its main functions are to regulate voluntary movements, including eye movements, and help with balance as well as posture.
There is a limbic region of the basal ganglia, which has multiple components (nucleus accumbens, ventral tegmental area, and ventral pallidum).
These areas are involved in cognitive and emotional behaviors and have a role in rewards and reinforcements. Because of this, it can be linked with addictive behaviors and the formation of habits.
Damage to the basal ganglia
Damage to the basal ganglia can result in tremors, involuntary muscle movements, abnormal posture, and links to movement disorders (Parkinson’s and Huntington’s disease).
In relation to the limbic system, the basal ganglia may also contribute to symptoms of depression (Stathis et al., 2007).
Research on the Limbic System
Several recent studies have furthered our understanding of the limbic system’s structure and function in both healthy and clinical populations:
- Rolls (2019) proposed there may be two separate limbic systems – one for emotion involving the amygdala, orbitofrontal cortex, anterior cingulate cortex, and one for memory involving the hippocampus and posterior cingulate cortex. This highlights the diverse roles of limbic regions.
- Neuroimaging research in Parkinson’s disease patients by Banwinkler et al. (2022) found limbic system changes correlated with symptoms like depression, impulse control disorders, cognitive decline, and even motor symptoms. This supports the role of the limbic system beyond emotion regulation.
- Structural MRI studies have identified limbic system abnormalities in psychiatric disorders. For example, Sahin et al. (2015) found damage to limbic regions in multiple sclerosis patients with emotional and memory deficits. White et al. (2008) found differences in cingulate, hippocampal, and amygdala structures in schizophrenic youth.
- Changes to limbic areas involved in emotion regulation have been identified in major depressive disorder (Maletic et al., 2007).
- In chronic traumatic encephalopathy, a neurodegenerative disease linked to repetitive head impacts, reduced volumes of the amygdala, hippocampus, and cingulate cortex were found in former NFL players (Lepage et al., 2018). This may indicate early neurodegeneration.
In summary, emerging research continues to reveal the widespread involvement of the limbic system in cognitive, emotional, behavioral, and neurodegenerative processes through diverse methodologies and clinical populations. This further emphasizes the central role of this system in brain function.
Treatment and Future Directions
As research continues to unravel the limbic system’s complexity, new treatment targets and approaches may emerge.
Deep brain stimulation has shown promise for treating certain limbic-mediated conditions like anxiety, PTSD, and addiction by modulating activity in regions like the amygdala and nucleus accumbens (Bari et al., 2014).
Antidepressant medications may also work by restoring limbic system physiology (Maletic et al., 2007).
Further research on the functional connectivity between limbic regions could aid in the diagnosis and monitoring of neurological and psychiatric disorders.
Advances in neuroimaging, optogenetics, and other techniques will enable more precise targeting of limbic areas involved in memory, emotion, and behavior. A better understanding of limbic development may uncover windows for early intervention.
Assessing and restoring limbic system function through multi-modal therapies could become an increasing focus in treating limbic-related disorders. Overall, the limbic system remains a promising target for developing new diagnostic, monitoring, and therapeutic approaches in clinical populations.
References
Banwinkler, M., Theis, H., Prange, S., & van Eimeren, T. (2022). Imaging the Limbic System in Parkinson’s Disease-A Review of Limbic Pathology and Clinical Symptoms. Brain Sciences, 12(9), 1248.
Bari, A., Niu, T., Langevin, J. P., & Fried, I. (2014). Limbic neuromodulation: implications for addiction, posttraumatic stress disorder, and memory. Neurosurgery Clinics of North America, 25 (1), 137–145.
Carmona, S., Vilarroya, O., Bielsa, A., Tremols, V., Soliva, J. C., Rovira, M., … & Bulbena, A. (2005). Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neuroscience letters, 389(2), 88-93.
DelBello, M. P., Adler, C. M., & Strakowski, S. M. (2006). The neurophysiology of childhood and adolescent bipolar disorder. CNS Spectrums, 11 (4), 298-311.
Grèzes, J., Berthoz, S., & Passingham, R. E. (2006). Amygdala activation when one is the target of deceit: did he lie to you or to someone else?. Neuroimage, 30 (2), 601-608.
Gulyaeva, N. V. (2019). Functional neurochemistry of the ventral and dorsal hippocampus: Stress, depression, dementia and remote hippocampal damage. Neurochemical Research.
Herman, J. P., McKlveen, J. M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., … & Myers, B. (2016). Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comprehensive physiology, 6(2), 603.
Jhaveri, D. J., Tedoldi, A., Hunt, S., Sullivan, R., Watts, N. R., Power, J. M., … & Sah, P. (2018). Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Molecular psychiatry, 23(3), 521-532.
Lepage, C., Muehlmann, M., Tripodis, Y., Hufschmidt, J., Stamm, J., Green, K., … & Koerte, I. K. (2019). Limbic system structure volumes and associated neurocognitive functioning in former NFL players. Brain Imaging and Behavior, 13 (3), 725-734.
Maletic, V., Robinson, M., Oakes, T., Iyengar, S., Ball, S. G., & Russell, J. (2007). Neurobiology of depression: an integrated view of key findings. International Journal of Clinical Practice, 61 (12), 2030-2040.
McEwen, B. S., Nasca, C., & Gray, J. D. (2016). Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology, 41(1), 3–23.
Morris, J. S., Frith, C. D., Perrett, D. I., Rowland, D., Young, A. W., Calder, A. J., & Dolan, R. J. (1996). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature, 383 (6603), 812-815.
Okun, M. S., Bowers, D., Springer, U., Shapira, N. A., Malone, D., Rezai, A. R., Nuttin, B., Heilman, K. M., Morecraft, R. J., Rasmussen, S. A., Greenburg, B. D., Foote, K. D. & Goodman, W. K. (2004). What’s in a “smile?” Intra-operative observations of contralateral smiles induced by deep brain stimulation. Neurocase, 10 (4), 271-279.
Pagliaccio, D., Luby, J. L., Bogdan, R., Agrawal, A., Gaffrey, M. S., Belden, A. C., … & Barch, D. M. (2015). Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. Journal of abnormal psychology, 124(4), 817.
Piretti, L., Pappaianni, E., Lunardelli, A., Zorzenon, I., Ukmar, M., Pesavento, V., Rumiati, R. I., Job, R., & Grecucci, A. (2020). The role of amygdala in self-conscious emotions in a patient with acquired bilateral damage. Frontiers in Neuroscience, 14, 677.
Ponirakis, G., Ghandi, R., Ahmed, A., Gad, H., Petropoulos, I. N., Khan, A., … & Woodruff, P. W. (2022). Abnormal corneal nerve morphology and brain volume in patients with schizophrenia. Scientific Reports, 12(1), 1870.
Rolls, E.T. (2019). The cingulate cortex and limbic systems for emotion, action, and memory. Brain Structure and Function, 224(9), 3001-3018.
Sahin, N., Selouan, R., Markowitz, C. E., Melhem, E. R., & Bilello, M. (2016). Limbic pathway lesions in patients with multiple sclerosis. Acta Radiologica, 57 (3), 341-347.
Sergerie, K., Chochol, C., & Armony, J. L. (2008). The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 32 (4), 811-830.
Stathis, P., Panourias, I. G., Themistocleous, M. S., & Sakas, D. E. (2007). Connections of the basal ganglia with the limbic system: implications for neuromodulation therapies of anxiety and affective disorders. Operative Neuromodulation, 575-586.
Tyng, C. M., Amin, H. U., Saad, M. N., & Malik, A. S. (2017). The influences of emotion on learning and memory. Frontiers in Psychology, 8, 1454.
Weissman, D. G., Lambert, H. K., Rodman, A. M., Peverill, M., Sheridan, M. A., & McLaughlin, K. A. (2020). Reduced hippocampal and amygdala volume as a mechanism underlying stress sensitization to depression following childhood trauma. Depression and anxiety, 37(9), 916-925.
White, T., Cullen, K., Rohrer, L. M., Karatekin, C., Luciana, M., Schmidt, M., Hongwanishkul, D., Kumra, S., Schulz, C. & Lim, K. O. (2008). Limbic structures and networks in children and adolescents with schizophrenia. Schizophrenia Bulletin, 34 (1), 18-29.
Xie, M., Yi, C., Luo, X., Xu, S., Yu, Z., Tang, Y., Zhu, W., Du, Y., Jia, L., Zhang, Q., Dong, Q., Zhu, W., Zhang, X., Bu, B., & Wang, W. (2011). Glial gap junctional communication involvement in hippocampal damage after middle cerebral artery occlusion. Annals of Neurology, 70(1), 121–132.
Yücel, M., Wood, S. J., Fornito, A., Riffkin, J., Velakoulis, D., & Pantelis, C. (2003). Anterior cingulate dysfunction: implications for psychiatric disorders?. Journal of Psychiatry and Neuroscience, 28 (5), 350.

